
However, if there is only one electron in an orbital it can have either a positive or negative spin. Using the Pauli exclusion principle we know that if there are two electrons in an orbital, one must be spin up (+ ½ ) and one must be spin down (- ½ ) to give them different quantum numbers. It pairs with the Aufbau principle to allow us to know what electron orbitals will be filled. The Pauli exclusion principle is important when determining the electron shell structure of an atom. Applications of the Pauli Exclusion Principle in Chemistry Since photons are bosons, however, they do not follow the Pauli exclusion rule. This is a violation of the Pauli exclusion rule. In one state, they all have the same quantum number. There can be many photons in one energy state. Therefore, all these particles will follow the Pauli exclusion principle. Commonly known fermions are electrons, protons, and neutrons. A fermion is an atomic particle that has a half-integer spin. Therefore, according to the definition of the Pauli exclusion principle, the orbital can only hold two electrons. The remaining quantum spin number only has two possible values. This definition originates from an orbital being defined by the first three quantum numbers. The principle also defines that each orbital can only have two electrons. The other quantum numbers are all interconnected. It can only have a value of + ½ or – ½ and these values are independent of all other quantum numbers. The spin quantum number is slightly different from the other quantum numbers because it is not dependent on them. A negative m s usually indicates spin down and is represented by a down-facing arrow. A positive m s usually indicates spin up and is represented by an upward pointing arrow.

The spin quantum number ( m s) was added to the previously discovered three quantum numbers ( n, l, m l) by the Pauli exclusion principle. Therefore, no two electrons will have the same four quantum numbers.
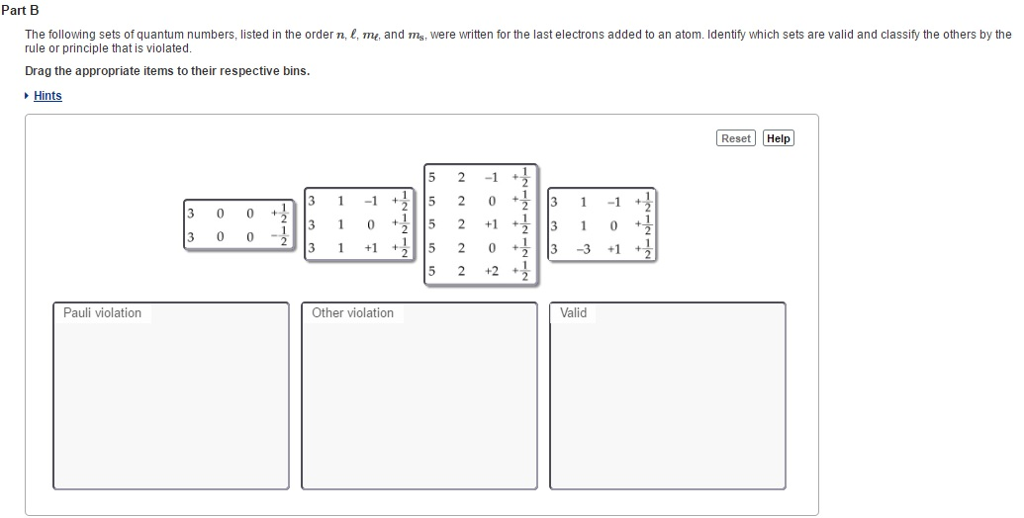
One electron will have m s =+ ½ and the other m s = – ½. So, in each electronic orbital (same n, l, and m l) there can be two electrons and they must have different spins. Every electron must have different quantum numbers. The Pauli Exclusion Principle states that in any atom no electron can have the same four electronic quantum numbers as another electron.


 0 kommentar(er)
0 kommentar(er)
